In a phase 3 study of 328 patients 6 to 17 years of age with functional constipation,
LINZESS demonstrated 2x greater improvement in bowel movement frequency over 12 weeks1
LINZESS was studied in a single, 12-week, double-blind, placebo-controlled, randomized, multicenter clinical trial in pediatric patients 6 to 17 years of age diagnosed with functional constipation. The primary endpoint was the change from baseline in SBM frequency rate during the 12-week treatment period.
Improvement in SBM frequency of 2.6 SBMs/week for patients treated with LINZESS vs 1.3 SBMs/week for patients treated with placebo (LSM change from baseline; P<0.0001)1,2
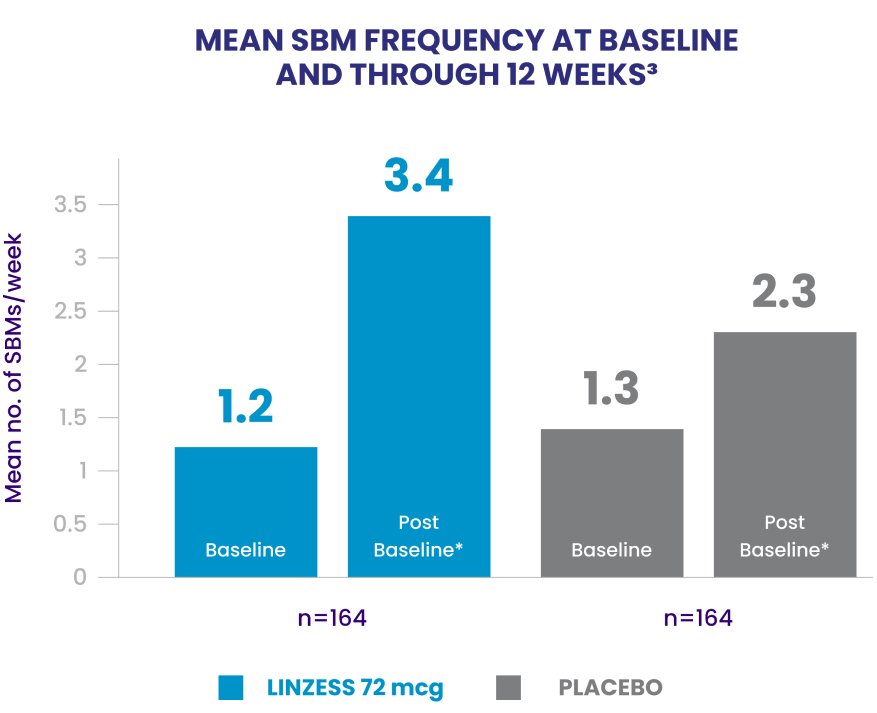
*Postbaseline data report the average over the 12-week treatment period.
LSM, least squares mean; SBM, spontaneous bowel movement.
LINZESS provided early and lasting improvement of bowel movement frequency through week 121
Patients taking LINZESS showed improvement in SBM frequency as early as week 1 and through week 121
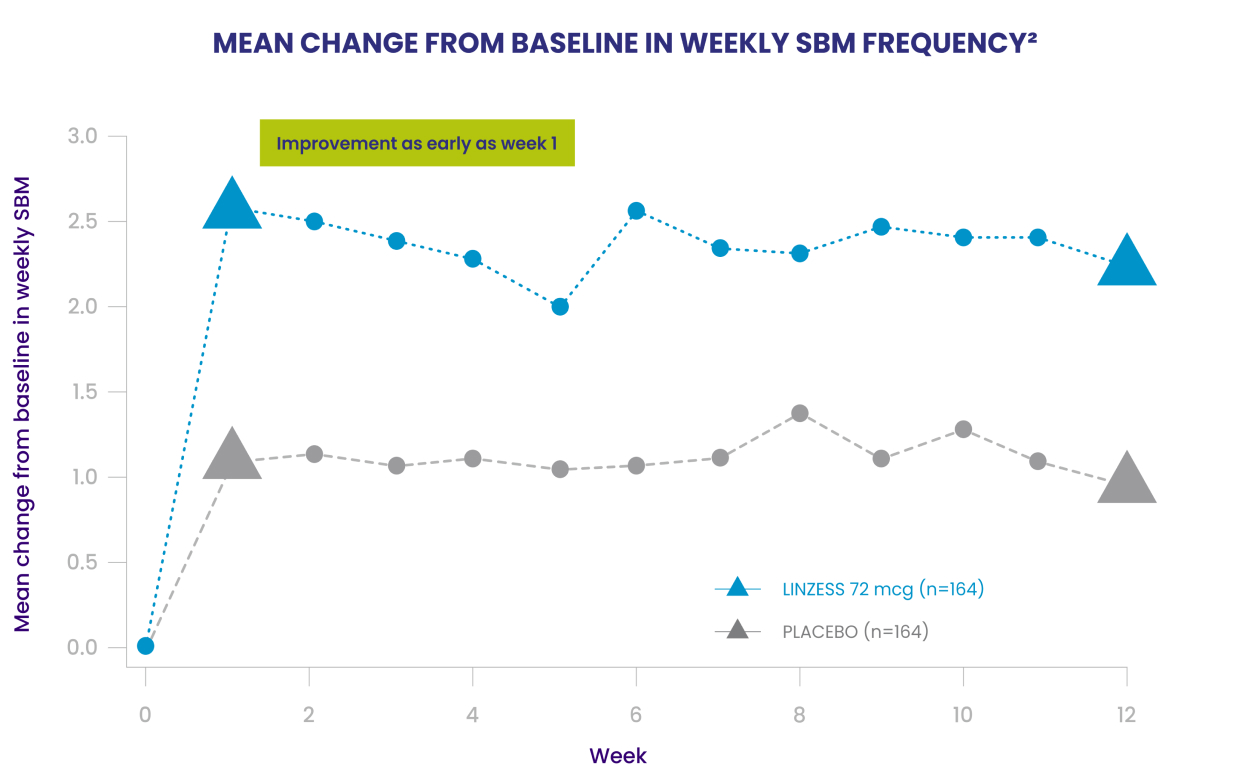
LINZESS was studied in 328 patients 6 to 17 years of age with functional constipation in a double-blind, placebo-controlled, randomized, multicenter phase 3 trial. The primary endpoint was the 12-week change from baseline in SBM frequency rate.
Patient characteristics:
- Mean age: 11 years (range, 6-17 years)
- Gender: 55% female
- Race/ethnicity: 45% identified as Hispanic or Latino; 70% identified as White, 26% as Black or African American, 2% as Asian, and 2% identified as another racial group
- All patients met modified Rome III criteria for child/adolescent functional constipation and reported <3 SBMs per week during the 2-week baseline period
- Patients with pediatric irritable bowel syndrome with constipation or fecal impaction were excluded
During the study period, patients were allowed to take bulk laxatives, fiber, stool softeners, probiotics, bisacodyl, and senna. Other laxatives bismuth, prokinetic agents, or other drugs to treat functional constipation were not allowed.



