Well-studied CIC safety profile1
LINZESS was evaluated in three phase 3, placebo-controlled safety trials involving more than 2,400 adults with CIC
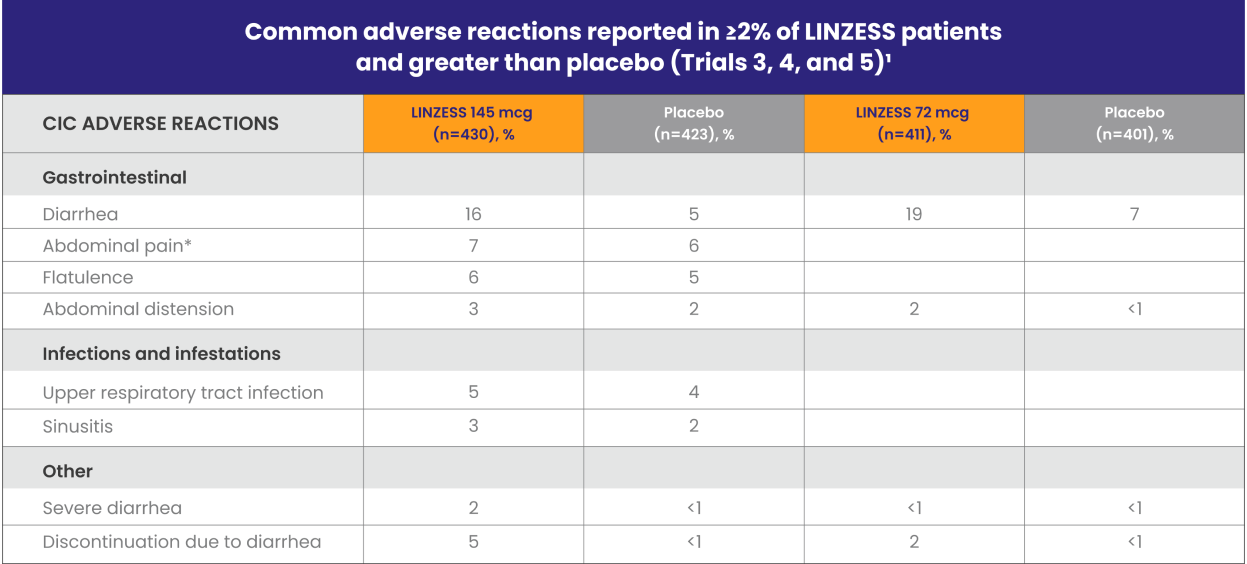
*“Abdominal pain” term includes the preferred terms: abdominal pain, upper abdominal pain, and lower abdominal pain.
In Trial 5, adverse reactions that occurred at a frequency of ≥2% in LINZESS 72 mcg–treated patients (n=411) and at a higher rate than placebo (n=401) were1:
- Diarrhea (LINZESS 72 mcg, 19%; LINZESS 145 mcg, 22%; placebo 7%)
- Abdominal distension (LINZESS 72 mcg, 2%; LINZESS 145 mcg, 1%; placebo <1%)
Diarrhea was the most commonly reported adverse reaction of LINZESS1
- In Trials 3, 4, and 5, 2% of the patients treated with LINZESS 145 mcg experienced severe diarrhea vs less than 1% in patients receiving placebo





